使用说明:
碧云天提供的各种基质胶应用方案仅供参考,实际须根据实验目的进行条件的优化。
1.基质胶的解冻、分装。
a.解冻:将基质胶小瓶埋于碎冰中并在4ºC冰箱避光过夜融化。请勿放置于冰箱门上或者经常需要开启的冰箱。一旦基质胶被解冻,请使用预冷的移液管轻柔的吹打混匀基质胶以确保基质胶分散均匀。
b.分装:使用预冷的移液管轻柔的吹打混匀基质胶以确保其分散均匀,然后根据后续实验所需量进行分装,然后置于-20ºC或以下温度避光保存。吸取基质胶过程中,如果吸头或移液管堵塞、吸取不精确时请更换吸头或移液管。
分装及后续实验操作基质胶时必须在冰浴上进行。
注1:基质胶在10ºC以上会开始凝胶化,所以实验过程中需要将基质胶一直置于冰上。同时,所有接触的移液管、吸头和离心管等耗材,以及用于稀释的培养液或者PBS等试剂都需4ºC预冷。
注2:一旦基质胶凝胶化,立刻放置在避光的4ºC冰上或冰箱中24-48个小时,凝胶化的基质胶可能会重新液态化,请勿直接转移至-20ºC以下冻存。
2. 基质胶的常规包被(Coating)方法。
基质胶有多种不同的包被方法,如薄胶法、厚胶法、薄胶包被法等,可根据实验目的选择合适的方法。
a. 薄胶法(Thin gel method):即由基质胶形成的凝胶厚约0.5mm,然后将细胞培养于该薄层凝胶上。薄胶法主要用于细胞贴壁和增殖,仅需要一层薄薄的蛋白层辅助,如原代细胞培养、细胞迁移和侵袭实验。
(a) 将基质胶置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
注:通常把标准型的Matrix-Gel™基质胶(8-13mg/ml)或低生长因子型的Matrix-Gel™基质胶(8-13mg/ml)的浓度适当稀释至1mg/ml以上,可根据实际情况进行调整。
(b) 将培养板置于冰上,按照50μl/cm
2向培养板/皿的生长表面加入基质胶。具体添加量可参考下表。
|
6-well plate |
12-well plate |
24-well plate |
48-well plate |
96-well plate |
35mm dish |
60mm dish |
100mm dish |
| Well area |
~9.6cm2 |
~4.5cm2 |
~2cm2 |
~0.8cm2 |
~0.32cm2 |
~8cm2 |
~21cm2 |
~55cm2 |
| Matrix-Gel™ volume |
480μl |
225μl |
100μl |
40μl |
16μl |
400μl |
1.05ml |
2.75ml |
(c) 将培养板/皿置于在37℃孵育30分钟以固化基质胶。
(d) (选做)吸出未结合的基质胶并使用不含血清的培养液轻柔漂洗后,即可使用。
注:需确保移液管的尖端不会刮伤凝胶层表面。
b. 厚胶法(Thick gel method):即由基质胶形成的凝胶厚约1-2mm,细胞在凝胶内部或者表面培养。厚胶法主要用于3D细胞培养,体外血管生成实验(成管试验, Tube assay),成环试验(Ring assay)如大鼠主动脉组织分化为毛细管样结构等。
(a) 将基质胶置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶基质至均匀状态。
(b) 向基质胶中加入细胞并使用预冷过的移液管进行悬浮处理。建议基质胶占比>67%。
(c) 将培养板置于冰上。按照150-200µl/cm
2向培养板/皿的生长表面加入与细胞混合后的基质胶(Mixed Matrix-Gel™)。具体添加量可参考下表。
|
6-well plate |
12-well plate |
24-well plate |
48-well plate |
96-well plate |
35mm dish |
60mm dish |
100mm dish |
| Well area |
~9.6cm2 |
~4.5cm2 |
~2cm2 |
~0.8cm2 |
~0.32cm2 |
~8cm2 |
~21cm2 |
~55cm2 |
| Mixed Matrix-Gel™ volume |
1.4-2.0ml |
675-900μl |
300-400μl |
120-160μl |
48-64μl |
1.2-1.6ml |
3.1-4.2ml |
8.25-11ml |
(d) 将培养板置于37℃孵育30分钟以固化基质胶。
(e) 可以根据实验需要添加培养液。细胞也可以培养在凝胶的表面。
c. 薄层包被法(Thin coating method):使用较低的基质胶浓度使仅形成混合的蛋白包被层,不形成凝胶(no
gel),然后将细胞培养于该薄层上。本方法可用于细胞粘附实验,如iPSC扩增或原代细胞扩增。
(a) 将基质胶置于冰中并在4℃融化,使用预冷的移液管混合基质胶至均匀状态。
(b) 使用不含血清的培养液或PBS将基质胶稀释到需要的浓度,通常在0.1mg/ml以上
注:需进行预实验确定最佳包被浓度。
(c) 培养板中加入稀释后的基质胶,加入的量应该足以轻易地覆盖整个生长表面,建议包被量为0.01-0.02mg/cm
2。然后室温孵育1小时以固化基质胶。
(d) 吸出未结合的基质胶并使用不含血清的培养液轻柔漂洗后,即可使用。
注:需确保移液管的尖端不会刮伤涂层表面。
3. 基质胶用于免疫缺陷小鼠(裸鼠, Nude mice)的皮下成瘤实验。
皮下成瘤实验一般选用4-8周龄的小鼠。伦理学通常要求接种肿瘤细胞数量不可超过1×10
7个/只。成瘤速度根据细胞的成瘤性、增殖速度、细胞接种量、小鼠品系等因素有所差异。药物干预周期一般为0.5-2个月。
a. 将
Matrix-Gel™基质胶(高浓度) (C0382/C0383或
C0386/
C0387)置于冰中并在4℃过夜融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
注:高浓度低因子基质胶的成瘤更快。
b.细胞收集:悬浮细胞直接收集细胞悬液,贴壁细胞用胰酶消化后收集细胞悬液。收集的细胞悬液300×g离心5分钟,PBS洗涤2次。PBS重悬细胞并计数,调整细胞密度为1×10
7-1×10
8个/ml。将细胞置于冰上待用。
注:收集的细胞宜在1小时内接种完毕。
c. 将细胞悬液与基质胶按照1:1的比例混匀,此时细胞密度为0.5×10
7-5×10
7个/ml。
注:细胞悬液与基质胶混合液需置于冰上以减缓细胞凋亡并防止基质胶凝固。
d. 使用
实验动物电动剃毛器(FS600)对小鼠注射部位皮肤进行脱毛,然后用酒精棉球进行消毒。
e. 用不带针头的1ml注射器将细胞悬液与基质胶混合液吸入注射器,再装上针头。
注:吸取混合液前可用注射器缓慢吹打以混匀细胞。
f. 进行皮下注射,接种体积通常为100µl/只。
注:注射过程应尽量缩短时间。
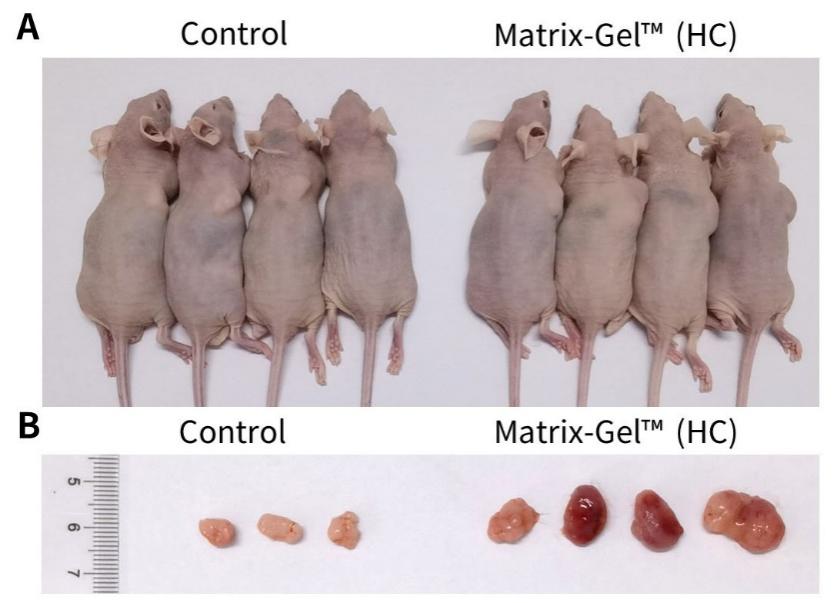
g. 注射完毕的小鼠继续饲养约1-3周可观察到较明显的瘤块。皮下成瘤实验的效果可参考图1。
4. 基质胶用于体外血管生成实验(以永生化HUVEC细胞系为例)。
a. 对HUVEC细胞进行饥饿处理:将完全培养液换成含0.2% FBS的ECM培养液,培养24小时。
注:建议使用3-5代状态较好且融合度为70-80%的HUVEC细胞。
b. 将Matrix-Gel™基质胶(标准型,
C0371/
C0372)置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
c. 使用ECM培养液将Matrix-Gel™基质胶稀释到需要的浓度。
注1:使用Matrix-Gel™基质胶进行血管形成实验,建议使用基质胶原液与ECM基础培养液的比例为2:1稀释。
注2:如选用Matrix-Gel™基质胶(低生长因子)进行成管实验,可考虑在培养液中添加适当的生长因子以刺激成管。
d. 将96孔板置于冰上,按照每孔50µl向96孔板中加入稀释后的基质胶,随后置于37℃孵育45分钟-1小时以固化基质胶。
e. 消化HUVEC细胞,用含10% FBS的ECM终止消化,使用ECM完全培养基重悬细胞,计数,调整细胞密度为2×10
5/ml。
注:对于HUVEC细胞,ECM培养基的体外血管生成效果较好,优选选择。如果使用DMEM培养基,细胞密度为2-4×10
5/ml,且成管时间可能要增加。不同的细胞系推荐的培养基、细胞密度和每孔的细胞量参见下表。
| Cell lines |
HUVEC |
RLSEC |
RAOEC |
U87 |
| Medium |
ECM complete medium |
DMEM-H+10% FBS |
DMEM-L+10% FBS |
DMEM-H+10% FBS |
| Cell density |
2×105/ml |
8×105/ml |
4×105/ml |
6×105/ml |
| Cell number/well |
10,000 |
40,000 |
20,000 |
30,000 |
f. 96孔板每孔加入50µl细胞悬液。
注1:HUVEC细胞容易沉底,易造成孔间细胞数量不一致,加样前需用移液器吹打混匀,避免细胞数量不一致影响成管效果。细胞数量不应少于1万个/孔,数量过少会导致无法形成连续的网络。
注2:加样时移液器吸头须轻靠培养孔侧壁缓慢加入,不要接触到凝胶。
注3:为提高实验准确性,请至少设置3个复孔。
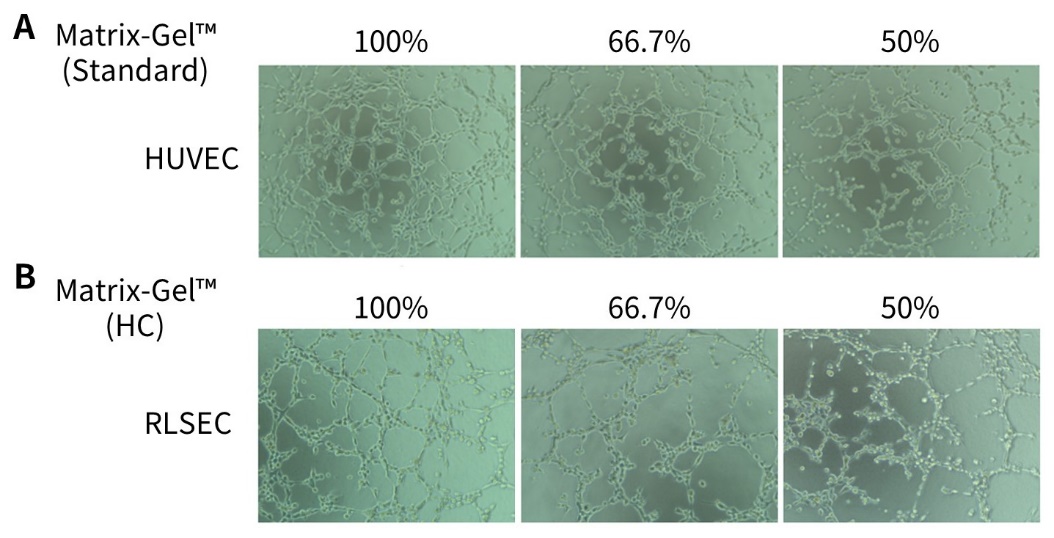
g. 将96孔板置于培养箱培养,4小时或24小时后观察结果。通常4小时后可见血管网络形成,成管时间与细胞状态密切相关。体外血管生成效果可参考图2。
注:血管形成后发生塌缩可能是培养时间过久,内皮细胞发生凋亡所致。
h. 在血管网络形成最佳时间,小心去除培养液,并用加入含活细胞染料
Calcein AM (钙黄绿素AM) (C2012)的DMEM培养液进行染色,用显微镜进行拍照记录。
5. 基质胶用于侵袭实验。
a. 基质胶用于Transwell小室的包被:
(a) 将Matrix-Gel™基质胶(标准型)置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
(b) 在冰上,将基质胶用无血清培养液按照1:8的比例进行稀释,例如取8µl基质胶加入64µl不含血清的培养液中,使用预冷的吸头混合至均匀状态。
注:常用的稀释比例为1:4、1:6、1:8,可根据实验具体情况调整稀释比例。
(c) 取60µl上述混合溶液垂直加入Transwell小室(Transwell chamber/Transwell
insert,即嵌套或上室)中,使其均匀平铺在底部,注意均匀铺胶,不要产生气泡。随后置于37℃孵育2小时。
注:Transwell
®是Corning公司的注册商标,类似的产品还有碧云天的BeyoGold™细胞小室系列产品(
FTW001-
FTW164)、Corning公司的BioCoat™ Matrigel Invasion Chambers和Falcon
® Multiwell Insert System、Greiner Bio-One公司的ThinCert™ Tissue Culture Inserts、Millipore的Millicell
® Standing Cell Culture Inserts等。
b. 细胞悬液的制备:
(a) (选做)对细胞进行12-24小时的饥饿处理。
(b) 消化细胞,用不含血清的培养液重悬,调整细胞密度为5-50万个/ml。
注:不同细胞的迁移能力不同,可设置一系列细胞密度梯度摸索合适的细胞密度。
c. 细胞接种:
(a) 取500µl含10%
FBS的培养液加入24孔板下室,用镊子将Transwell小室置于24孔板内。
注:小室的放置过程经常有气泡产生,一旦产生气泡,下层培养液的趋化作用就减弱甚至消失,因此需特别留心。一旦出现气泡,需将小室提起,去除气泡后重新放置。
(b) 取200µl细胞悬液轻柔加入包被凝胶的Transwell小室上侧。
注:须确保移液管的尖端不会刮伤凝胶层表面。
(c) 将24孔板置于培养箱中培养24-48小时。
注:接种细胞1-2小时后,可对培养板进行检查,确保没有大气泡产生。
d. 细胞固定、染色:
(a) 取出Transwell小室,去除培养液,用棉签轻轻擦拭基质胶及细胞。
(b) 在24孔板干净的孔中加入600µl
4%多聚甲醛固定液(P0099),将小室放入固定20-30分钟。
(c) 弃固定液,PBS洗涤小室1次。
注:需避免触碰小室底部。
(d) 在24孔板干净的孔中加入适量
结晶紫染色液(C0121),将小室放入染色5-10分钟。
(e) 取出小室,PBS洗涤3次。
注:需避免触碰小室底部。
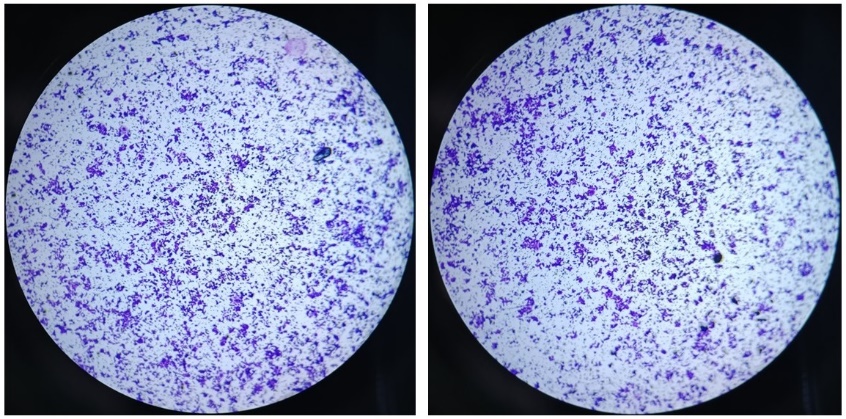
(f) 适当风干后,显微镜下观察并计数,侵袭实验最终染色效果可参考图3。
6. 基质胶用于类器官(Organoid)培养。
a. 将Matrix-Gel™基质胶(类器官用)置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
b.取组织(动物组织、肿瘤组织),充分清洗修剪后转移至干净的容器中进行剪切。再加入合适的组织消化液进行消化(例如:肠道组织使用2.5~5mM EDTA溶液4ºC消化30~60分钟,其它组织使用消化酶37℃消化30~60分钟)。待显微镜下观察出现完整的腺体或者细胞团即可终止消化。收集细胞悬液进行清洗后,用适量的类器官培养液调整细胞密度后再加入相应比例的Matrix-Gel™基质胶(类器官用),混合均匀。Matrix-Gel™基质胶(类器官用)的比例通常大于70%。
c. 取出培养板,立即取适量基质胶与单细胞悬液的混合溶液滴加到培养板中心,避免混合溶液接触培养孔侧壁,并立即置于37ºC培养箱中孵育10-30分钟以固化基质胶(若细胞团体积较大可倒置培养板进行固化)。
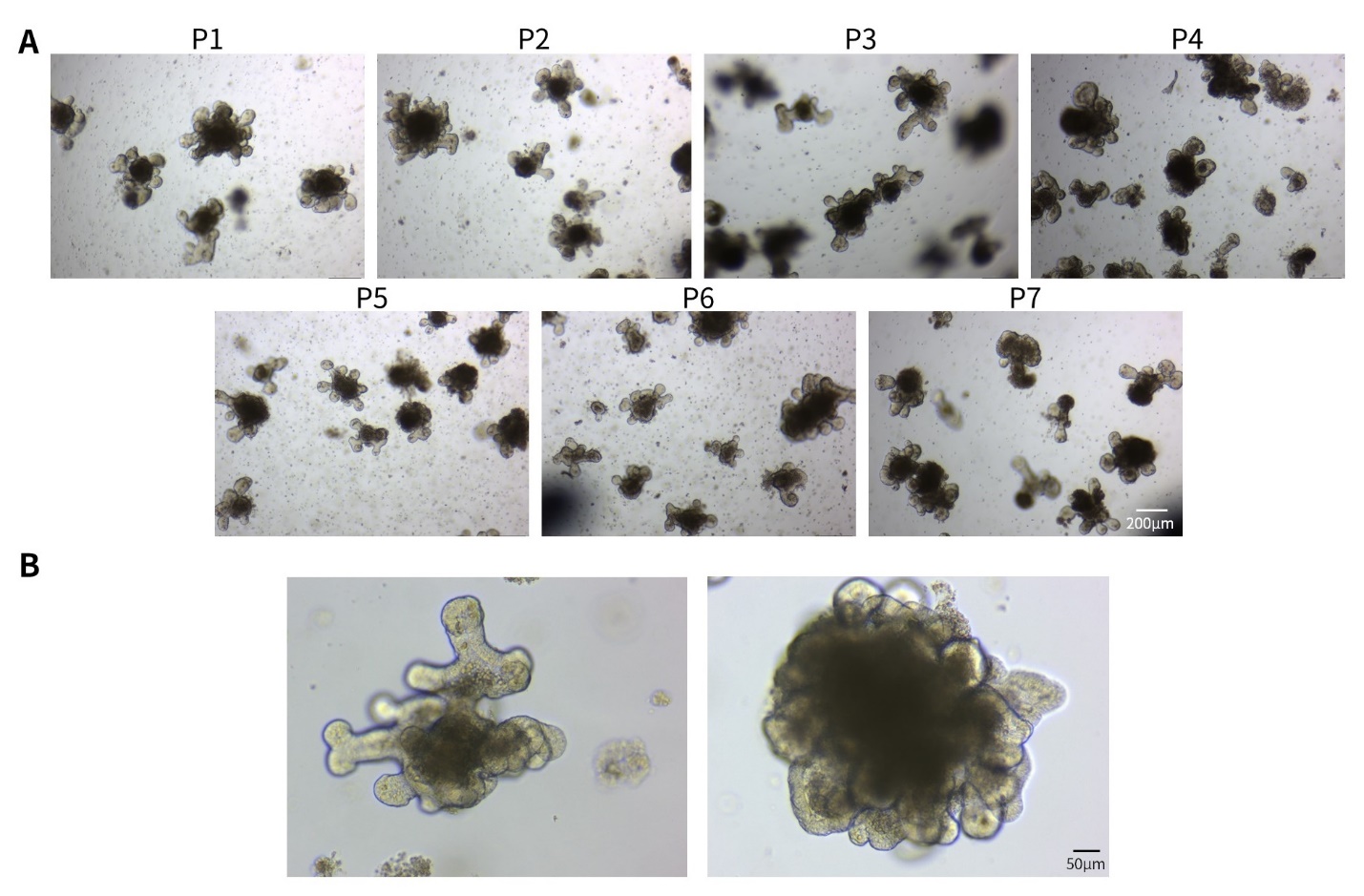
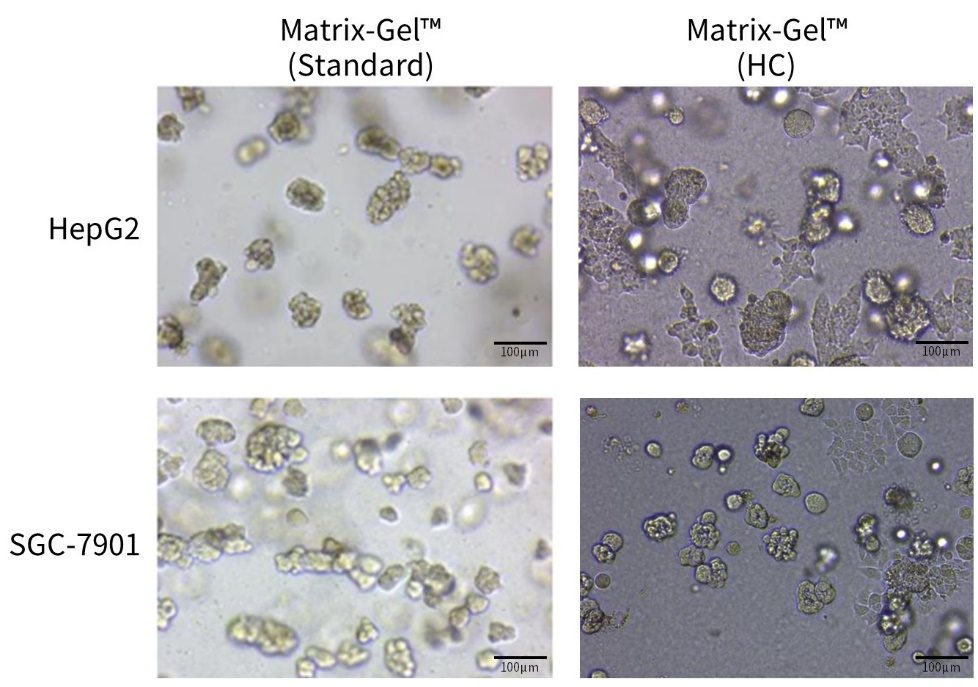
d.待基质胶凝固后,向24孔板中加入500µl相应的类器官培养液,置于培养箱中培养,并每隔一定时间更换培养液。类器官培养效果可参考图4、5。
e. 待基质胶凝固后,向24孔板中加入500µl相应的类器官培养液,置于培养箱中培养,并每隔一定时间更换培养液。类器官培养效果可参考图4、5。
7. 肿瘤类器官药敏实验。
a. 将扩增好足够量的肿瘤类器官(实验组)以及正常类器官(对照组)用4℃预冷的基础培养液进行重悬,缓慢机械吹打,促进胶液化溶解,并保持类器官结构完整;或者通过
BeyoTryp™ Express Enzyme(C0191/
C0192)消化获得单细胞悬液。
b. 进行类器官的培养。培养板可以是96孔板或384孔板。
c. 类器官形成后(如果是机械吹打,类器官将在传代24小时后就会形成;如果是酶消化,类器官会在3-5天后形成),每孔分别加入不同种类、不同浓度的待筛选的抗肿瘤药物。
d. 用
CellTiter-Lumi™发光法3D细胞活力检测试剂盒(C0061/
C0062)检测类器官的活力或通过荧光染料和高内涵筛选(High-content screening,
HCS)进行活细胞成像,测定肿瘤类器官对各种药物的敏感性。
8. 基质胶用于3D细胞培养。
注:以96孔板为例,对于其它细胞培养材料,可进行相应的调整。也可直接参考“2b.厚胶法”。
a. 根据细胞的特点,按正常培养条件培养细胞。
b. 消化并收集细胞,用不含血清的培养液重悬细胞,计数,根据实验所需吸取细胞悬液,300×g离心5分钟,弃上清后置于冰上。
c. 将Matrix-Gel™基质胶(标准型或高浓度)置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
d. 用适量的基质胶混合液轻柔重悬细胞沉淀,注意避免产生气泡。基质胶的比例大于70%,推荐重悬后的细胞密度为每10μl重悬液中含5000个细胞,重悬后置于冰上,重悬时间不应超过30秒以避免基质胶过早凝固。
注:细胞密度可根据实验具体情况进行调整。
e. 用适量的基质胶混合液轻柔重悬细胞沉淀,注意避免产生气泡。推荐重悬后的细胞密度为每10μl重悬液中含5000个细胞,重悬后置于冰上,重悬时间不应超过30秒以避免基质胶过早凝固。
注:细胞密度可根据实验具体情况进行调整。
f. 将培养板置于冰上,以8μl/孔垂直滴加到96孔板中心部位,然后用移液器吸头慢慢均匀铺开。将96孔板小心倒扣,随后置于37℃孵育30分钟以固化基质胶。
g. 基质胶凝固后,向96孔板中以每孔60μl加入相应预热的含10% FBS的培养液,边缘空白孔补充无菌水。
注:如有待测化合物可同时添加。
h. 将培养板置于37℃培养箱培养,每天观察细胞生长和3D结构形成的情况。
i. 每隔4天(第4、8、12天)更换新的培养液。
j. 待细胞生长至理想状态时,进行分析。3D肿瘤球形成实验的效果可参考图6。
9. 基质胶用于诱导多能性干细胞(Induced Pluripotency Stem Cell, iPSC)培养。
注1:iPSC培养过程中不建议使用抗生素,抗生素会影响细胞的活性和分化潜能。
注2:iPSC培养环境应与其它细胞隔离,两次传代后检测支原体。
a. 基质胶用于多孔板的包被:
(a) 将Matrix-Gel™基质胶(干细胞用)置于冰中并在4℃融化,使用预冷的移液管或吸头混合基质胶至均匀状态。
(b) 在冰上,将基质胶与预冷DMEM培养液按照1:80或1:100的比例混匀,例如取1ml基质胶加入80ml或100ml 预冷DMEM培养液中,使用预冷的移液管在冰上混合至均匀状态。
注:可根据实验具体情况调整稀释比例。
(c) 将6孔板置于冰上,按照每孔1ml向6孔板中加入稀释后的基质胶。
(d) 将6孔板置于37℃孵育过夜。
注:一般情况下,孵育1小时即可使用,但过夜孵育的细胞培养效果更好,包被的培养板可4℃存放1周。
(e) 使用前吸出未结合的基质胶。
注:需确保移液管的尖端不会刮伤涂层表面。
b. Y-27632 (ROCK抑制剂)溶液的配制:将
Y-27632 (SC0326)溶于PBS中,配制成10mM的Y-27632溶液,例如取2.47mg Y-27632溶于1ml PBS中,即得1ml 10mM Y-27632溶液。
注:Y-27632的工作浓度为10µM。
c. 配制含Y-27632的人胚胎干细胞培养液(如mTeSR™1或TeSR™-E8™)中:取Y-27632溶液与mTeSR™1培养液按照1:1000的比例混匀,例如取10µl Y-27632溶液加入10ml
mTeSR™1培养液中,即得10ml含10µM Y-27632的mTeSR™1培养液。
d. iPSC复苏:
(a) 将冻存的iPSC置于37℃水浴迅速解冻,将细胞溶液转移至离心管中,用DMEM培养液冲洗冻存管两次并转移至离心管中,与管中细胞合并,室温300×g离心5分钟。
(b) 弃上清,用2ml含Y-27632的mTeSR™1培养液重悬iPSC,随后将细胞悬液转移至基质胶包被好的6孔板的1个孔中,置于培养箱中培养。
注:建议将每支复苏的细胞(约100万个)转移至6孔板的1个孔中培养使细胞存活率最大化。
(c) 每天对iPSC进行换液,将含Y-27632的mTeSR™1培养液更换为不含Y-27632的mTeSR™1培养液。ES细胞的培养效果图可参考图7。
e. iPSC传代:
注:iPSC细胞生长至单层后会迅速分化并死亡,为保持其活性和多能性,需在长满前进行传代。
(a) 去上清,用1ml PBS洗涤1次,加入1ml适当的细胞消化液如
BeyoTryp™ Express Enzyme (C0191/
C0192)或Accutase。
(b) 将培养板转移至培养箱中孵育3分钟。显微镜下观察,大部分细胞脱落,若细胞仍附着,可以用手轻轻拍打培养板底部使细胞脱落。
(c) 将消化下来的细胞转移至离心管中,用DMEM培养液洗涤培养板表面两次并转移至离心管中,与管中的细胞合并,室温300×g离心5分钟。
(d) 弃上清,用含Y-27632的mTeSR™1培养液进行重悬,随后将细胞悬液转移至基质胶包被好的6孔板中,置于培养箱中培养。
(e) 每天对iPSC进行换液,将含Y-27632的mTeSR™1培养液更换为不含Y-27632的mTeSR™1培养液。
f. iPSC冻存:
(a) 冻存液配制:20% FBS+10% DMSO+70% DMEM或90% FBS+10% DMSO,推荐使用碧云天
细胞冻存液(C0210)。
(b) 参考细胞消化液或冻存液的相关步骤进行细胞消化。
(c) 计数,弃上清,加入适量冻存液,以每管1ml冻存液(约100万个细胞)分装至冻存管中。
(d) 将细胞冻存管放置于可逐步降低温度的装置如
BeyoCool™细胞冻存盒(FCFC012/
FCFC015/
FCFC021/
FCFC026)内并放置在-80℃冰箱内,确保冷却速度约为1℃/分钟。
(e) 放置在-80℃冰箱内约24小时后转移至液氮中保存。
常见问题:
一、关于基质胶使用的基本问题。
1. Matrix-Gel™基质胶可以储存在-80℃吗?
是的。Matrix-Gel™基质胶可以储存在-80℃。建议用户将整瓶的Matrix-Gel™基质胶进行分装,储存于聚丙烯(PP)或其它可以耐受超低温条件材质的小管中,方便保存和使用。
2. Matrix-Gel™基质胶可以反复冻融吗?
建议用户第一次融化后按照单次用量进行分装保存,须避免反复冻融。
3. 未使用完的Matrix-Gel™基质胶应该怎样保存?
不建议保存已经与培养液或PBS等缓冲液混合过但未使用完的基质胶。
4. 应该如何对Matrix-Gel™基质胶移液操作?
推荐使用预冷的注射器、移液管或吸头进行操作。吸液时不要触及瓶子底部;分液时切忌过快、用力过猛。
5. 使用Matrix-Gel™基质胶时,需要将培养板/皿、吸头和离心管等耗材预冷吗?
是的。因为Matrix-Gel™基质胶在高于10℃的条件下会开始成胶,推荐操作时使用预冷的移液管、吸头和离心管。
6. Matrix-Gel™基质胶能快速聚合吗?
Matrix-Gel™基质胶在22-35℃时会快速聚合成胶。
7. 怎样稀释Matrix-Gel™基质胶?
使用冰上预冷的不含血清的培养液或者PBS对Matrix-Gel™基质胶进行稀释。
8. Matrix-Gel™基质胶包被过的培养板/皿可以储存多长时间呢?
包被过的培养板/
皿最好当天使用。如暂不使用,在37℃培养箱中最多存放7天。保存时,基质胶表面需要使用不含血清的培养液均匀覆盖,以保持湿润。
9. Matrix-Gel™基质胶的最低成胶浓度是多少?
Matrix-Gel™基质胶最低成胶浓度为3mg/ml。不同的实验目的需要不同的基质胶浓度,应该根据具体的实验需求确定。不同批次间的Matrix-Gel™基质胶浓度有差异,应该根据最终工作浓度(mg/ml)算出需要加入的稀释液体(如PBS或无血清培养液)的量。用于体内研究的Matrix-Gel™基质胶,为了避免成胶不完全,最终工作浓度不应低于4mg/ml。
10. Matrix-Gel™基质胶胶块在体内可以维持多长时间?
可以在体内维持至少一周的时间。
11. 为什么Matrix-Gel™基质胶在37℃成胶,而在4℃时却呈液体状态?
Matrix-Gel™基质胶是一种从小鼠EHS肿瘤中提取的基底膜基质,主要包括以下成分:层粘连蛋白、IV型胶原蛋白、巢蛋白、硫酸类肝素蛋白多糖、表皮生长因子、类胰岛素生长因子及其它生长因子。这些蛋白构成了基质胶的基本结构。在22-37℃时,大分子间可以通过化学键结合,促使基质胶形成凝胶。而在低温条件(如4℃)下,由于没有足够的能量促使化学键结合,所以基质胶呈现液体状态。
12. 为什么Matrix-Gel™基质胶很粘稠?
基质胶的蛋白浓度越高,胶体越粘稠。如果浓度高于13.0mg/ml,基质胶会显得非常厚重。Matrix-Gel™基质胶产品在未稀释前都会比较粘稠。高浓度的Matrix-Gel™基质胶不稀释可以直接用于细胞培养或者体内注射,也可以稀释至任意蛋白浓度范围并当成标准型Matrix-Gel™基质胶产品进行使用。具体稀释浓度根据用户实验需求确定。
除因为产品本身浓度高而粘稠外,基质胶的状态还与运输过程中温度的变化和储藏条件有关。整个运输过程中必须使用干冰冷藏。如果储藏Matrix-Gel™基质胶的冰箱带有自动除霜功能,冰箱除霜过程中升温,可能使基质胶成胶。所以,勿将Matrix-Gel™基质胶储藏于此类冰箱中。为保证Matrix-Gel™基质胶的使用效果,冻融次数应该尽可能减少。收到新的Matrix-Gel™基质胶后,请立即按照单次用量进行分装。每次融化操作,Matrix-Gel™基质胶都应该放置于冰上。如果Matrix-Gel™基质胶在成胶状态时被冻住,再次融化时将不能恢复成液体。
13. 为什么细胞没有贴壁?Matrix-Gel™基质胶也脱落了?
首先需要检查细胞的接种浓度是否过高,基质胶的用量应等同于细胞培养体系中培养液的用量。如果基质胶被稀释到过低的浓度,形成的胶体容易从组织培养器皿表面分离。
14. 未稀释的Matrix-Gel™基质胶中出现的沉淀应该怎样处理?
4℃低速离心,去除沉淀物。
15. Matrix-Gel™基质胶的折射率是多少?
在20℃时,Matrix-Gel™基质胶的折射率约为1.3406-1.3407,相对折射率约为1.0056 (同等条件下,水的折射率为1.333)。
16. Matrix-Gel™基质胶会有自发荧光吗?
Matrix-Gel™基质胶是一种蛋白混合物,经过透析处理后溶解在DMEM培养液中。为防止微生物污染,培养液中添加了庆大霉素。所以基质胶可能引发荧光的组分包括其中的蛋白质成分,维生素成分以及庆大霉素(氨基糖苷类抗生素)。如果需要使用荧光检测细胞生长状态,建议设置对照实验,在所需要的波长条件下进行对比,以便排除背景荧光。
二、关于基质胶的应用问题。
1. 什么情况下需要使用不含酚红的Matrix-Gel™基质胶?
对于涉及颜色检测的实验,推荐使用不含酚红的Matrix-Gel™基质胶,如使用荧光染料对内皮细胞成管(Tubulogenesis)的定量,而对子宫内膜细胞培养就必须使用不含酚红的Matrix-Gel™基质胶。此外,酚红与一些非甾体雌激素结构相似,有显著的雌激素活性,在实验动物体内可能具有干扰内分泌与荷尔蒙代谢的能力,故不含酚红的Matrix-Gel™基质胶也可以应用于一些体内应用研究。此外,酚红是一种潜在的内分泌干扰物,有可能会干扰实验动物体内激素的自然产生和代谢能力。
2. 高浓度Matrix-Gel™基质胶可以用于哪些实验?
高浓度Matrix-Gel™基质胶可用于3D细胞培养、裸鼠体内成瘤实验等体内应用研究。高浓度Matrix-Gel™基质胶的蛋白浓度高,和肿瘤细胞一起皮下注射到小鼠体内后保持其完整性并保持原位,以便进行原位分析或未来肿瘤的取样。
3. 哪些情况下应该选用薄胶法/厚胶法?3D细胞培养有哪些应用?
薄胶法主要用于细胞贴壁和增殖,如原代细胞培养仅需要一层薄薄的蛋白层辅助而非蛋白基质;厚胶法主要用于3D细胞培养,成环试验(Ring
assay)如大鼠主动脉组织分化为毛细管样结构,以及进行细胞侵袭实验等。3D细胞培养实验,主要是用于研究细胞与细胞间的相互作用以及复杂结构等。
4. Matrix-Gel™基质胶可以用于诱导ES/iPS细胞分化的研究吗?
可以,实验表明Matrix-Gel™基质胶可以用于ES/iPS细胞的分化研究,建议使用干细胞用的
Matrix-Gel™基质胶(C0391/
C0392)。
5. 如何从Matrix-Gel™基质胶中收获细胞?
推荐使用中性蛋白酶如
分散酶(ST2339)或
BeyoTryp™ Express Enzyme (C0191/
C0192)来收获培养在Matrix-Gel™基质胶中的细胞。中性蛋白酶和BeyoTryp™ Express
Enzyme相比胰蛋白酶、胶原蛋白酶或其它蛋白水解酶能够更温和有效地获得单细胞悬液,不会损伤细胞或分裂细胞表面蛋白。对于需要继续接种培养或生物检测的细胞,使用中性蛋白酶或BeyoTryp™ Express
Enzyme不会产生损伤,此外中性蛋白酶或BeyoTryp™ Express Enzyme也可以用于组织的分离。也可使用相应的细胞复苏溶液进行代谢实验和RNA回收实验,建议在4℃使用细胞复苏溶液进行非酶反应的细胞收获。
其它方法:降低温度至4-6℃使基质胶解聚,这需要一定的时间并且仅适合一部分应用;或者离心以破坏基质胶结构。
6. 使用Matrix-Gel™基质胶培养的细胞,如果需要进行切片或者免疫组织化学及免疫荧光检验,该怎样固定呢?如何避免解聚?
可以使用4%多聚甲醛进行固定,如4%多聚甲醛配制在PBS中,室温固定20分钟。某些情况下,在固定后可能出现解聚的情况,可以加入1%戊二醛(戊二醛作为固定剂,常用于电镜检测)。如果需要进行免疫荧光检验,加入戊二醛后,会出现明显的背景荧光。为了解决这一问题,建议用户在固定之后,使用NaBH4进行淬灭。NaBH4极易起泡,进行该步骤时,必须在水平操作台上小心操作,避免晃动,尽量减少气泡的形成。另外,也可以尝试使用较低浓度(如0.1%到0.5%)的戊二醛进行固定,浓度越低,背景荧光信号越少。
7. 提取过程会引起层粘连蛋白变性吗?
不会引起层粘连蛋白变性。
三、基质胶中的成分问题。
1. Matrix-Gel™基质胶中含有DNA或RNA吗?
是的,Matrix-Gel™基质胶没有经过DNase或RNase消化处理,可能会含有痕量的DNA、RNA。此外,因为基质胶中含有痕量的RNA,进行RNA分析时,应设一个Matrix-Gel™基质胶(不接种细胞的对照组)。
2. Matrix-Gel™基质胶中含有血管内皮生长因子(VEGF)和金属蛋白酶(MMPs)吗?
标准型的Matrix-Gel™基质胶中含有5.0-7.5ng/ml的血管内皮生长因子(VEGF),低生长因子的Matrix-Gel™基质胶中的VEGF含量为1.0-1.5ng/ml。另外,可能还含有小鼠肿瘤细胞来源的痕量的金属蛋白酶(MMPs)。
3. Matrix-Gel™基质胶中含有LDEV吗?
不含。Matrix-Gel™基质胶经免疫方法及PCR方法检测,并不含有乳酸脱氢酶增高病毒(LDEV)或者乳酸脱氢酶增生病毒(LDHV),同时也未发现细菌、真菌和支原体。
4. Matrix-Gel™基质胶含中有尿素吗?
不含。
5. Matrix-Gel™基质胶中使用的什么缓冲液?
低糖DMEM (1g/L),其中包含50µg/ml庆大霉素。
6. Matrix-Gel™基质胶中含有纤维连接蛋白(Fibronectin)吗?
是的,经Western Blot检测,在Matrix-Gel™基质胶中发现了微量的纤维连接蛋白(Fibronectin)。
7. Matrix-Gel™基质胶中含有玻璃体结合蛋白(Vitronectin)吗?
某些组织中可能含有微量的血液,因此Matrix-Gel™基质胶中可能会有痕量的玻璃体结合蛋白(Vitronectin)。
8. Matrix-Gel™基质胶中还有什么别的物质?
Matrix-Gel™基质胶中还可能含有浓度小于0.02%的三氯甲烷,以及肿瘤细胞的产生的其它未知蛋白或分子。
参考文献:
1. Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Adv Drug Deliv Rev. 2014. 79-80:3-18.
2. Hughes CS, Postovit LM, Lajoie GA. Proteomics. 2010. 10(9):1886-1890.
3. Biederer T, Scheiffele P. Nat Protoc. 2007. 2(3):670-6.
4. Yu X, Sidhu JS, Hong S, Faustman EM. Toxicol Sci. 2005. 84(2):378-93.
5. Roskelley CD, Desprez PY, Bissell MJ. Proc Natl Acad Sci U S A. 1994. 91(26):12378-82.
6. Debnath J, Muthuswamy SK, Brugge JS. Methods. 2003. 30(3):256-68.
7. Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, et al. Biochemistry. 1982. 21(24):6188-6193.
8. Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, et al. Biochemistry. 1986. 25(2):312-8.
9. Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, et al. Exp Cell Res. 1992. 202(1):1-8.
10. McGuire PG, Seeds NW. J Cell Biochem. 1989. 40(2):215-227.
相关产品:



 微信在线咨询
微信在线咨询













 微信在线咨询
微信在线咨询











